Research
Our laboratory focuses on the development of innovative and environmentally friendly chemical and material conversion processes. We develop homogeneous and heterogeneous catalysts, as well as continuous flow science technologies and equipment, based on synthetic organic chemistry. From these, we attempt to innovate in fine chemical synthesis, such as pharmaceuticals, agrochemicals, semiconductor materials, and high-performance chemicals, by utilizing biomass, carbon dioxide, hydrogen, and other resources to reduce reliance on fossil fuels. We conduct education and research across science, engineering, pharmaceutical sciences, and agriculture. We will advance research and development to achieve green material conversion and realize the SDGs and a carbon-neutral society.

Our laboratory promotes research based on the following six topics as the central pillars with cooperation each other.
Organic Reactions Explored in Water

Water is a sine qua non of sustainability in global environment and living things. The pivotality of water intrigues us in molecular science.
All living things, including human being, are made up of water. A number of chemical reactions performed in a living organism made up its metabolism, maintaining chemical and ionic gradients (homeostasis). Most enzymes provide a sequestered and somehow hydrophobic environment to exclude water, where chemical reaction is regulated tightly. It means that in vivo enzymatic reaction achieves fine synthesis in water.
To synthesize pharmaceutical or chemicals, organic reactions have been long deemed to be conducted in organic solvents, owing to the compatibility and immiscibility between water molecules and many reagents or reactive species. Most organic solvents are, however, harmful to human body and environment, which urged chemists to seek “greener” solvent alternatives (non-toxic, inexpensive, and benign to environment). We believe that water is the most attractive candidate up to now. In addition, water sometimes provides us with a potential to achieve unique reactivity and selectivity that cannot be observed in conventional organic solvents. Our group has extensively engaged in the development of multifarious catalytic systems that are water-compatible. One of our fruits, Lewis Acid‒Surfactant-combined Catalyst (LASC) is regarded to be a highly efficient catalyst that works in water, forming hydrophobic micelle along with water-tolerant Lewis acidity. A variety of reactions such as aldol reactions are efficiently promoted in the presence of LASCs in water even though organic solvent is not used at all.
One of our biggest concerns is exploring catalytic asymmetric reactions in water toward efficient synthetic methodologies of optically active compounds. To date, we have developed enantioselective Mannich-type reactions catalyzed by chiral zinc catalyst, followed by enantioselective hydroxymethylation reactions, Michael-type addition reaction of thiols, and enantioselective protonation reactions catalyzed by chiral scandium catalyst in water. Furthermore, we have spotlighted zero-valent metals, and metal oxides or hydroxides that have not been used so far. Their outstanding catalytic activities in aqueous environments are discovered, leading to a set of highly stereoselective bond-forming reactions such as allylation reactions of aldehydes or ketones, boron conjugate addition reactions. These reactions do not proceed (or are not selective) at all in the absence of water, which underscores the positive effect of water that involves in reaction mechanism rather than the role as an alternative solvent.
Since the advent of organic chemistry, Friedrich Wӧhler’s urea synthesis in 1828, it has been systematically cultivated in organic solvents. In contrast, our intensive researches have disclosed a number of examples on new reactivity and selectivity observed in chemical reactions performed in water that are not obtained in organic solvents. Now the curtain rises to exploit water positively as a solvent. Since independently-developed “organic chemistry in water” cannot be written by the traditional organic chemistry cultivated in organic solvents, “organic chemistry in water” wants systemizing. An enzyme, an ultimate tool created by nature, would play a prominent role in chemistry systemized in water, whereas our ultimate goal is to create catalytic activity superior to enzymatic one.
Topics
- Asymmrmetric Conjugate Addition
Development of Highly Stereoselective Catalysts in Organic Synthesis
Development of selective transformation reactions to obtain only target molecules without formation of any by-product is a very important research topic from a viewpoint for saving energy and reducing chemical wastes. Therefore, development of new catalysts overcoming those problems is highly desired. Recently, our group is interested in asymmetric catalysis to prepare optically active intermediates as a single enantiomer, and has developed chiral zirconium, niobium, copper, and silver catalysts for various catalytic carbon-carbon bond forming reactions. Now, we are focusing on development of chiral alkaline earth metal catalysts, which are ubiquitous, safe and cheap elements, and on development of metal amides as strong basic catalysts for reactions of less acidic substrates.
Topics
- Development of metal amides as catalysts for organic reactions
- Development of catalytic asymmetric reactions using alkaline earth metal complexes
- Development of methodologies for activation of less reactive substrates
- Development of catalytic reactions using organosuperbases
Novel Heterogeneous Catalysts
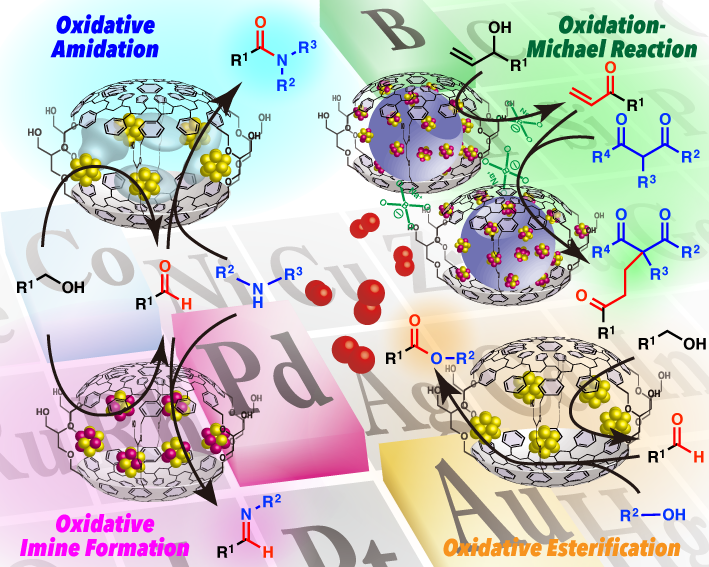
We immobilized various metal catalysts into polystyrene based organic co-polymer with cross-linking moieties. Our catalysts can be synthesized based on two key techniques, microencapsulation (MC) and polymer incarceration (PI).
Polymer and metal catalysts can be dissolved in good solvent of polymer and precipitates of both polymer and metal catalysts generate after addition of poor solvent for polymer. We call this phenomenon as microencapsulation (MC). This polymer precipitates can be heated under neat condition to make cross-link of polymer side chains and completely heterogeneous catalysts are obtained. In this solid, metal catalysts can be stabilized by weak but multiple interactions between metal and benzene rings in polymer. In addition, polymer backbone physically envelopes metal catalysts to prevent leaching from polymer matrix. We call this method as polymer incarceration (PI).
Based on these techniques, we could immobilize various toxic and precious metal complexes such as, Sc, Os, Pd, and Ru. Recently we are focusing on encapsulation of metal nanoparticles including Au, Pt, Pd, Rh etc. to realize highly reactive and robust heterogeneous catalysts. We can achieve various types of reaction, such as aerobic oxidation, hydrogenation, cross-coupling, and even asymmetric reaction. Moreover these catalysts can be applied to tandem processes and flow systems. These catalysts can be reused by simple filtration and metal leaching into products are suppressed to realize truly practical and environmentally benign organic synthesis that fulfill the criteria of green sustainable chemistry.
Reviews
- Tandem Oxidative Processes Catalyzed by Polymer-Incarcerated Multimetallic Nanoclusters with Molecular Oxygen
Miyamura, H.; Kobayashi, S.
Acc. Chem. Res. , 47, 1054 (2014). DOI: 10.1021/ar400224f - Polymer-Incarcerated Metals: Highly Reactive, Recoverable, and Multifunctional Nanocluster Catalysts for Organic Synthesis
Kobayashi, S.; Miyamura, H.
Aldrichimica Acta , 46, 3-19 (2013). - Polymer-Incarcerated Metal(0) Cluster Catalysts
Kobayashi, S.; Miyamura, H.
Chem. Rec. , 10, 271 (2010). 10.1002/tcr.201000026
Topics
- Aerobic oxidation reactions catalyzed by Au nanoparticle catalysts (PI-Au)
- Size effect of Au nanoparticles on reactivity and selectivity
- Direct ester, amide and imine synthesis using molecular oxygen
- Cooperative catalytic system of metal nanoparticles and quinone derivatives as artificial enzyme
- Asymmetric synthesis using chiral metal nanoparticles
- Tandem reactions using multifunctional heterogeneous catalysts
- Application to flow systems
- Metal nanoparticle catalysts with NHC active cross-linker

Development of new methodologies for organic reactions
Development of new methodologies for activating target molecules efficiently is highly desired. Our group is focusing on transformation reactions promoted under photo-irradiation conditions toward environmental friendly organic synthesis, and developing useful reactions based on photo-activation of substrates and catalysts. Our group is also interested in development of new functional groups for effective activation of less reactive substrates in catalysis.
Topics
- Development of organic reactions using fluorenylidene group
- New Reactions Utilizing Carbon Dioxide
- Selective C-H Functionalization via Single Electron Transfer Process (SET)
- Visible Light Photoredox Catalysis
Flow "Fine" Synthesis
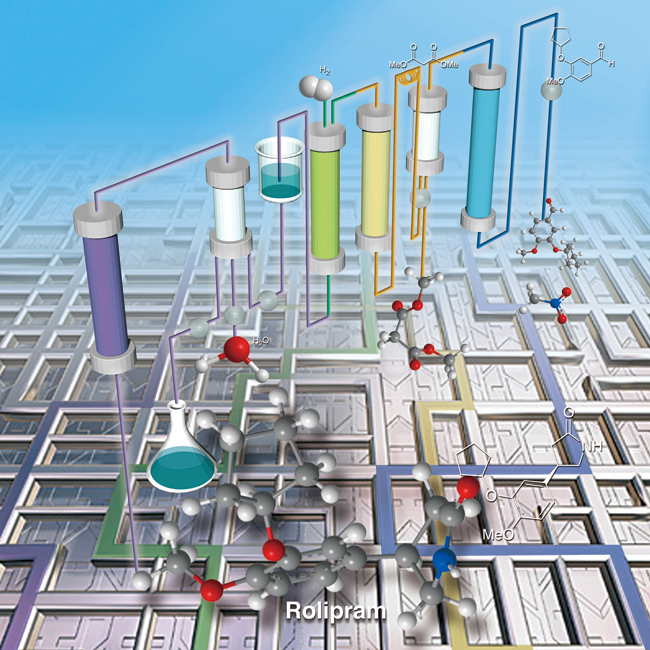
In the area of fine chemical production, chemical products such as medicines, agrichemicals and functional materials are often synthesized in batch systems. However, those methods are not suitable for view point of GSC (Green Sustainable Chemistry) due to the production of much amount of chemical wastes.
On the other hand, synthesizes through flow systems are usually preferred in bulk chemical production and petrochemistry i.e. Haber-Bosch ammonia production. We are considering that these flow systems should be applied for fine chemical production, especially drugs.
There are many advantages in flow systems compering to batch systems. Firstly, this system provides high economic efficiency and easy production control. Also it can save space, time and energy. Secondly, it is safe. Finally, we could prepare the multi-step reaction systems just connecting different flows, this causes avoidance of isolation and purification of intermediates. These advantages fully meet the criteria of GMP (Good Manufacturing Practice) policy. In recent, US FDA commented“right now, manufacturing experts from the 1950s would easily recognize the pharmaceutical manufacturing processes of today. It is predicted that manufacturing will change in the next 25 years as current manufacturing practices are abandoned in favor of cleaner, flexible, more efficient continuous manufacturing.”
We are considering that the above flow chemistry needs strategically new idea of protocols to achieve high atom efficiency, and we are now working on the development of new chemical reactions and effective heterogeneous/solid catalysts suitable for the continuous flow syntheses.
Reviews
- Flow “Fine” Synthesis: High Yielding and Selective Organic Synthesis by Flow Methods
S. Kobayashi,
Chem. Asian J., Early View , (2015). DOI: 10.1002/asia.201500916 - Asymmetric Carbon-Carbon Bond Formation under Continuous Flow Conditions with Chiral Heterogeneous Catalysts,
T. Tsubogo, T. Ishiwata, S. Kobayashi,
Angew. Chem. Int. Ed., 52, 6590-6604 (2013). DOI: 10.1002/anie.201210066
Topics
- Multistep Continuous Flow Synthesis of (R)- and (S)-Rolipram
- Continuous Flow Hydrogeneation of Nitro Compounds using a Polysilane Catalyst
Synthesis of Bioactive Compounds

As a practical applied example of a catalyst and the reaction system which we developed, we synthesized various bioactive compounds. As for most of these compounds, the configuration of structure was unclear when we began the synthesis. Asymmetric synthesis can produce any stereoisomers. Therefore, we could determine the actual configurations of these compounds or found the error of reported structure, at the same time finish the total synthesis.
Topics
- Revised Stereochemistry of Ceramide-Trafficking Inhibitor HPA-12 by X-ray Crystallography Analysis